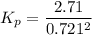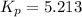For the reaction: 2 A (g) + B (s)= 2 C(s) + D (g)
At 298 K in a 10.0 L vessel, the equilibrium...

Chemistry, 29.05.2020 19:03 amberskids2
For the reaction: 2 A (g) + B (s)= 2 C(s) + D (g)
At 298 K in a 10.0 L vessel, the equilibrium values are as follows: 0.721 atm of A, 4.18 mol of B, 6.25 mol of C, and 2.71 atm of D. What is the value of the equilibrium constant?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2


Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
Questions




Arts, 10.03.2020 07:54










Spanish, 10.03.2020 07:54

Mathematics, 10.03.2020 07:54

Mathematics, 10.03.2020 07:54

Computers and Technology, 10.03.2020 07:54



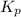 (equilibrium constant) is referred to as the partial pressure of product divided by the partial pressure of reactant with each pressure term raised to power that is equal to its stoichiometric coefficient in balanced equation
.
(equilibrium constant) is referred to as the partial pressure of product divided by the partial pressure of reactant with each pressure term raised to power that is equal to its stoichiometric coefficient in balanced equation
.![K_p = \dfrac{[D]}{[A]^2}](/tpl/images/0670/4630/dcbe2.png)