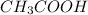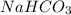Chemistry, 28.05.2020 15:00 stefancvorovic1
During an experiment vinegar was added to baking soda. The following observations were recorded.
Observation 1: The container with the reaction mixture feels cooler.
Observation 2: Gas bubbles are released.
Based on the observation, justify the type of change (physical or chemical) that took place.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
During an experiment vinegar was added to baking soda. The following observations were recorded.
Questions


English, 19.11.2020 07:00

Geography, 19.11.2020 07:00





Chemistry, 19.11.2020 07:00

History, 19.11.2020 07:00




Physics, 19.11.2020 07:00


Mathematics, 19.11.2020 07:00

World Languages, 19.11.2020 07:00


Mathematics, 19.11.2020 07:00

Mathematics, 19.11.2020 07:00