
Chemistry, 29.05.2020 00:58 NickWinston261
What would be the pH at the equivalence point of a 0.01M solution of strong acid titrated using 45mL of 0.02Mof a strong base? *

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 11:30
The dashed segment of the plotted experiment in the graph in the l
Answers: 3
You know the right answer?
What would be the pH at the equivalence point of a 0.01M solution of strong acid titrated using 45mL...
Questions

History, 15.07.2019 16:00

Physics, 15.07.2019 16:00


Mathematics, 15.07.2019 16:00

Spanish, 15.07.2019 16:00



Advanced Placement (AP), 15.07.2019 16:00

Mathematics, 15.07.2019 16:00



Mathematics, 15.07.2019 16:00


Mathematics, 15.07.2019 16:00

Physics, 15.07.2019 16:00


History, 15.07.2019 16:00



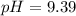
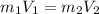
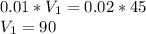
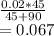
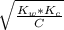
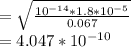
![pH = -log [H^+]](/tpl/images/0669/5500/9b8d2.png)



