
Chemistry, 28.05.2020 17:58 sofiaarmy12
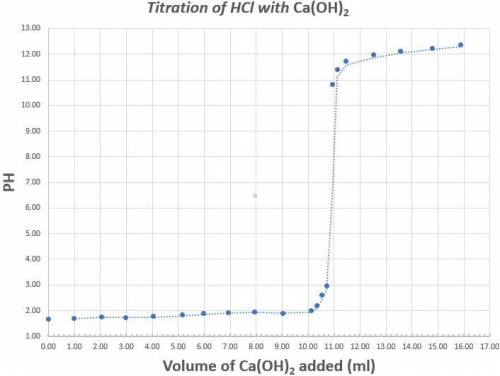
Read the graph to the nearest tenth for both pH and volume of calcium hydroxide. 25.0ml of a 1.70x10-4M solution of Hydrochloric acid was titrated with Calcium hydroxide. The above graph was generated when the Hydrochloric acid was titrated with Calcium hydroxide. Determine the concentration (in M) of the Calcium hydroxide. What is the coefficient of the scientific notation answer for the concentration of Calcium Hydroxide.
Determine the percent error if the known concentration of calcium hydroxide is 6.30x10-4M. (Do not put your answer in scientific notation).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

You know the right answer?
Read the graph to the nearest tenth for both pH and volume of calcium hydroxide. 25.0ml of a 1.70x10...
Questions

Mathematics, 19.02.2021 19:10


Social Studies, 19.02.2021 19:10


World Languages, 19.02.2021 19:10


Mathematics, 19.02.2021 19:10


Mathematics, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10



Mathematics, 19.02.2021 19:10

Social Studies, 19.02.2021 19:10


Mathematics, 19.02.2021 19:10

History, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10



