
Chemistry, 27.05.2020 19:01 laneycasey9058
One liter of oxygen gas at standard temperature and pressure has a mass of 1.43 g. The same volume of hydrogen gas under these conditions is 0.089 g. If both volumes contain the same number of gas particles (according to Avogadro's hypothesis), how can this difference in mass be explained?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3


Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1

Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3
You know the right answer?
One liter of oxygen gas at standard temperature and pressure has a mass of 1.43 g. The same volume o...
Questions

Mathematics, 11.02.2021 01:00

Biology, 11.02.2021 01:00

Chemistry, 11.02.2021 01:00

Chemistry, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00


Mathematics, 11.02.2021 01:00


Mathematics, 11.02.2021 01:00

History, 11.02.2021 01:00

English, 11.02.2021 01:00

Chemistry, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00



History, 11.02.2021 01:00

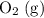 is larger than that of
is larger than that of 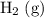 (by a factor of about
(by a factor of about 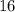 .) Therefore, the mass of the
.) Therefore, the mass of the 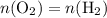 .
. is different from the number of gas particles
is different from the number of gas particles 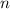 in it. In particular, if all particles in this gas have a molar mass of
in it. In particular, if all particles in this gas have a molar mass of  , then:
, then: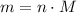 .
.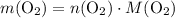 .
.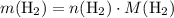 .
.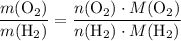 .
.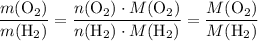 .
. :
: 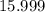 .
.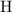 :
: 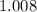 .
. .
.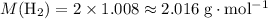 .
.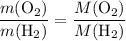 :
: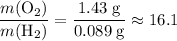 .Right-hand side:
.Right-hand side:  .
.

