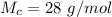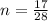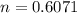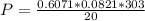Chemistry, 27.05.2020 16:57 urstruulyemily
A reaction between liquid reactants takes place at in a sealed, evacuated vessel with a measured volume of . Measurements show that the reaction produced of carbon monoxide gas. Calculate the pressure of carbon monoxide gas in the reaction vessel after the reaction. You may ignore the volume of the liquid reactants. Be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 09:00
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2
You know the right answer?
A reaction between liquid reactants takes place at in a sealed, evacuated vessel with a measured vol...
Questions




Mathematics, 13.11.2020 22:50




Biology, 13.11.2020 22:50

Computers and Technology, 13.11.2020 22:50

History, 13.11.2020 22:50


Computers and Technology, 13.11.2020 22:50

Mathematics, 13.11.2020 22:50

Mathematics, 13.11.2020 22:50

English, 13.11.2020 22:50

Business, 13.11.2020 22:50


Mathematics, 13.11.2020 22:50

Social Studies, 13.11.2020 22:50

Mathematics, 13.11.2020 22:50

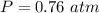
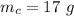
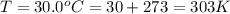
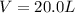
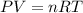
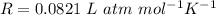
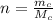
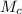 is the molar mass of carbon monoxide which is a constant with value
is the molar mass of carbon monoxide which is a constant with value