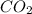Chemistry, 27.05.2020 19:09 alydiale584
A 0.533 g sample of a carbonate salt is added to a flask. The only thing you know is that per formula unit, there is only one carbonate anion, but the identity of the cation (or cations) is unknown. To determine the identity of the cations, 15.0 mL of 1.00 M sulfuric acid is added to the flask. After the resulting reaction completes, excess acid remains. The remaining solution is titrated with 0.5905 M NaOH(aq) and reaches the equivalence point when 29.393 mL of base is added. What is the identity of the compound?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
A 0.533 g sample of a carbonate salt is added to a flask. The only thing you know is that per formul...
Questions


Mathematics, 20.09.2020 07:01



Spanish, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01

Physics, 20.09.2020 07:01

English, 20.09.2020 07:01




History, 20.09.2020 07:01


Mathematics, 20.09.2020 07:01

Health, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01

History, 20.09.2020 07:01



English, 20.09.2020 07:01

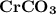
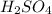 added = 15 × 1 × 10⁻³
added = 15 × 1 × 10⁻³ 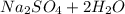
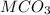 ------>
------> 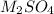 +
+ 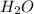 +
+