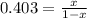Chemistry, 28.05.2020 04:57 jacquetpaul1969
A key step in the extraction of iron from its ore is FeO(s) + CO(g) ⇋ Fe(s) + CO2(g) Kp =0.403 at 1000°C. What are the equilibrium partial pressures of carbon monoxide and carbon dioxide when 1.00 atm of carbon monoxide and excess iron(II) oxide react in a sealed container at 1000°C. write Kp expression, do ICE chart with pressures, 0.287 atm CO2 and 0.713 atm CO

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
A key step in the extraction of iron from its ore is FeO(s) + CO(g) ⇋ Fe(s) + CO2(g) Kp =0.403 at 10...
Questions


Medicine, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20


Mathematics, 17.02.2021 04:20




Mathematics, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20

Social Studies, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20

Biology, 17.02.2021 04:20

English, 17.02.2021 04:20

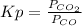 = 0.403
= 0.403