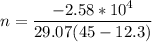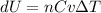Nitrogen gas in an expandable container is cooled from 45.0 C to 12.3 C with the pressure held constant at 2.58 ∗ 105 Pa. The total heat liberated by the gas is 2.58 ∗ 104 J. Assume that the gas may be treated as ideal. Find (a) the number of moles of gas; (b) the change in internal energy of the gas; (c) the work done by the gas. (d) How much heat woul

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Nitrogen gas in an expandable container is cooled from 45.0 C to 12.3 C with the pressure held const...
Questions

Mathematics, 26.07.2020 05:01

Mathematics, 26.07.2020 05:01




Social Studies, 26.07.2020 05:01

Chemistry, 26.07.2020 05:01


Mathematics, 26.07.2020 05:01

History, 26.07.2020 05:01






Mathematics, 26.07.2020 05:01

Chemistry, 26.07.2020 05:01



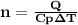
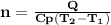
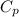 is the specific heat at constant pressure of Nitrogen gas which is = 29.07 J/mol/K
is the specific heat at constant pressure of Nitrogen gas which is = 29.07 J/mol/K