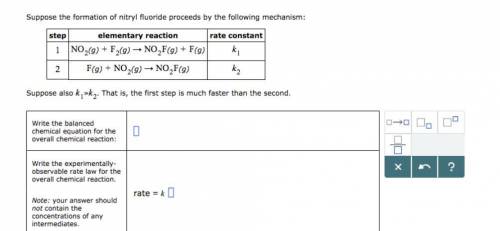
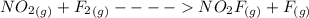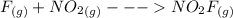Suppose the formation of nitryl fluoride proceeds by the following mechanism: step elementary reaction rate constant (g) (g) (g) (g) (g) (g) (g) Suppose also ≫. That is, the first step is much faster than the second. Write the balanced chemical equation for the overall chemical reaction: Write the experimentally-observable rate law for the overall chemical reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1


Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Suppose the formation of nitryl fluoride proceeds by the following mechanism: step elementary reacti...
Questions

English, 02.07.2019 11:00

Mathematics, 02.07.2019 11:00

Health, 02.07.2019 11:00

Chemistry, 02.07.2019 11:00


Arts, 02.07.2019 11:00





Social Studies, 02.07.2019 11:00



Mathematics, 02.07.2019 11:00

English, 02.07.2019 11:00

History, 02.07.2019 11:10

English, 02.07.2019 11:10




![r = \frac{ k [NO_2]^2 [F_2]}{[NO_2F]}](/tpl/images/0668/0949/108c7.png)