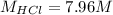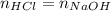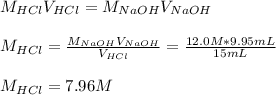Chemistry, 28.05.2020 01:06 psychocatgirl1
Concentrated hydrochloric acid is a solution that is 37.5% mass per unit volume HCl(aq) in water. An old bottle of HCl has an unknown concentration. What is the concentration of hydrochloric acid, [HCl], in the old bottle, if 9.95 mL of 12.0 M NaOH(aq) is required to reach the equivalence point when added to 15 mL of acid?What is the concentration of HCl(aq)?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
Concentrated hydrochloric acid is a solution that is 37.5% mass per unit volume HCl(aq) in water. An...
Questions

Mathematics, 20.01.2021 21:50

Mathematics, 20.01.2021 21:50

Mathematics, 20.01.2021 21:50


English, 20.01.2021 21:50

English, 20.01.2021 21:50

Mathematics, 20.01.2021 21:50

Mathematics, 20.01.2021 21:50

Chemistry, 20.01.2021 21:50

Arts, 20.01.2021 21:50


Mathematics, 20.01.2021 21:50


French, 20.01.2021 21:50


Social Studies, 20.01.2021 21:50

Mathematics, 20.01.2021 21:50


Social Studies, 20.01.2021 21:50

Mathematics, 20.01.2021 21:50