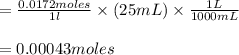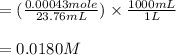Chemistry, 27.05.2020 21:07 gabriella80
2. 25.0mL aliquots of the solution in problem 1 are titrated with EDTA to the calmagite end point. A blank containing a small measured amount of Mg2 requires 2.12mL of the EDTA to reach the end point. An aliquot to which the same amount of Mg2 is added requires 25.88mL of the EDTA to reach the end point. a. How many mL of EDTA are needed to titrate the Ca2 ion in the aliquot

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
2. 25.0mL aliquots of the solution in problem 1 are titrated with EDTA to the calmagite end point. A...
Questions


Mathematics, 01.09.2019 19:50


Mathematics, 01.09.2019 19:50



Mathematics, 01.09.2019 19:50

History, 01.09.2019 19:50

Mathematics, 01.09.2019 19:50

Health, 01.09.2019 19:50






Mathematics, 01.09.2019 19:50

Mathematics, 01.09.2019 19:50

Mathematics, 01.09.2019 19:50


Biology, 01.09.2019 19:50