
Chemistry, 27.05.2020 15:57 Batzs3rdacct
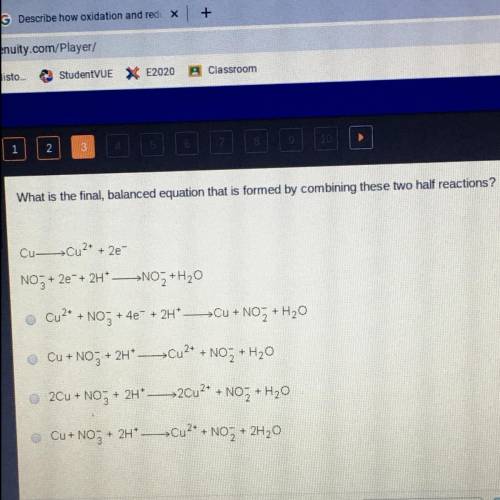
What is the final, balanced equation that is formed by combining these two half reactions?
Cu-Cu2+ + 2e-
NO3 + 2e + 2H* —>N0Z+H20
Cu2+ + NO3 + 4e- + 2H+ -->Cu +
u + NO3 + H20
Cu + NO3
+ 2HCu2+ + NO2 + H20
2Cu + NO3 +
+ 2H+ ->20u2+ +
+ NO 2 + H20
Cu+ NO3 +
+ 2H+
Cu2+ + NO + + 2H20

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

You know the right answer?
What is the final, balanced equation that is formed by combining these two half reactions?
Cu-...
Cu-...
Questions

History, 15.10.2020 09:01

English, 15.10.2020 09:01


Mathematics, 15.10.2020 09:01


English, 15.10.2020 09:01

Biology, 15.10.2020 09:01

Mathematics, 15.10.2020 09:01

Mathematics, 15.10.2020 09:01



Mathematics, 15.10.2020 09:01

Biology, 15.10.2020 09:01




Mathematics, 15.10.2020 09:01

Computers and Technology, 15.10.2020 09:01


Social Studies, 15.10.2020 09:01



