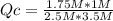Chemistry, 27.05.2020 11:57 antionette1
HCl (g) + NaOH (aq) <-> NaCl (aq) + H2O (l). What is the Q value for the equation and which direction will the reaction shift given that K = 0.5? , [HCl] = 2.5 M, [NaOH] = 3.5 M, [NaCl] = 1.75 M, [H2O] = 1 M

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
You know the right answer?
HCl (g) + NaOH (aq) <-> NaCl (aq) + H2O (l). What is the Q value for the equation and which di...
Questions





Mathematics, 06.08.2021 01:00


Mathematics, 06.08.2021 01:00

Mathematics, 06.08.2021 01:00


English, 06.08.2021 01:00



Computers and Technology, 06.08.2021 01:00







![Qc=\frac{[C]^{c}*[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/0667/0235/1eb27.png)
![Qc=\frac{[NaCl]*[H_{2}O] }{[HCl]*[NaOH]}](/tpl/images/0667/0235/1a295.png)