
Chemistry, 26.05.2020 23:06 TheOriginalMeyah
Which equation shows how to calculate how many grams of koh would be needed to fully react with 4mol Mg(OH)2? The balanced reaction is: MgCl2 + 2KOH = Mg(OH)2 + 2KCl
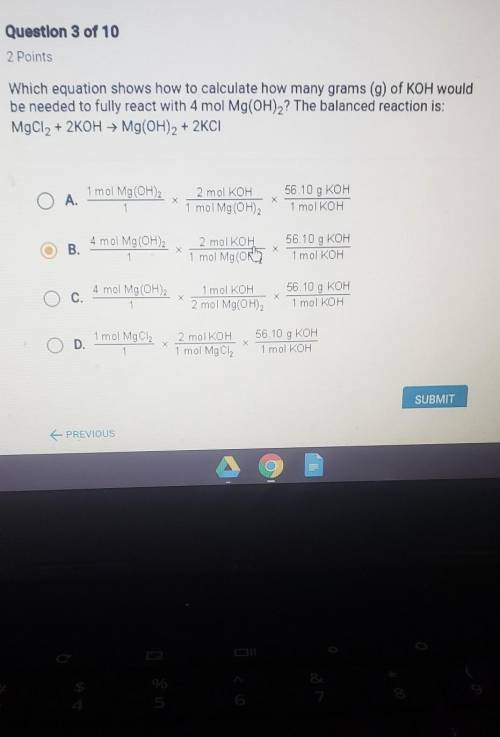

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Which equation shows how to calculate how many grams of koh would be needed to fully react with 4mol...
Questions



Chemistry, 10.11.2020 07:50

History, 10.11.2020 07:50

Chemistry, 10.11.2020 07:50

Mathematics, 10.11.2020 07:50

Chemistry, 10.11.2020 07:50



Mathematics, 10.11.2020 07:50

English, 10.11.2020 07:50



Engineering, 10.11.2020 07:50

Mathematics, 10.11.2020 07:50





Chemistry, 10.11.2020 07:50



