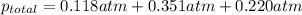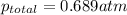Chemistry, 25.05.2020 05:57 lamooothegoat
What is the total pressure in atmospheres of a mixture of three gases with partial pressures of 0.118 atm, 35.6 kPa, and 167 mm Hg?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1
You know the right answer?
What is the total pressure in atmospheres of a mixture of three gases with partial pressures of 0.11...
Questions



Computers and Technology, 20.12.2020 22:00






Mathematics, 20.12.2020 22:00





Mathematics, 20.12.2020 22:00

History, 20.12.2020 22:00




Mathematics, 20.12.2020 22:00

Mathematics, 20.12.2020 22:00

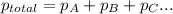
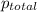 =total pressure of gases = ?
=total pressure of gases = ?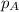 = partial pressure of A = 0.118 atm
= partial pressure of A = 0.118 atm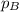 = partial pressure of B = 35.6 kPa = 0.351 atm (1kPa =0.0098atm)
= partial pressure of B = 35.6 kPa = 0.351 atm (1kPa =0.0098atm)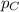 = partial pressure of C = 167 mm Hg = 0.220 atm (1mmHg=0.0013 atm)
= partial pressure of C = 167 mm Hg = 0.220 atm (1mmHg=0.0013 atm)