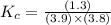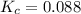Chemistry, 23.05.2020 21:58 melinda12ms
In the reaction NO + NO2 ⇌ N2O3, an experiment finds equilibrium concentrations of [NO] = 3.8 M, [NO2] = 3.9 M, and [N2O3] = 1.3 M. What is the equilibrium constant Kc for this reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1


Chemistry, 23.06.2019 11:30
Bridget is in science class. her teacher gives her two unknown substances and asks her to determine their relative ph. she places a piece of red litmus paper into both substances. the litmus paper turns purple when she places it into substance i. the litmus paper turns blue when she places it into substance ii. a. substance i is a neutral substance and substance ii is an acid. b. substance i is a neutral substance and substance ii is a base. c. substance i is an acid and substance ii is a base. d. substance i is a base and substance ii is a neutral substance.
Answers: 1
You know the right answer?
In the reaction NO + NO2 ⇌ N2O3, an experiment finds equilibrium concentrations of [NO] = 3.8 M, [NO...
Questions

Mathematics, 26.08.2020 19:01




English, 26.08.2020 19:01

Physics, 26.08.2020 19:01





Mathematics, 26.08.2020 19:01

Mathematics, 26.08.2020 19:01



Chemistry, 26.08.2020 19:01

Biology, 26.08.2020 19:01

Biology, 26.08.2020 19:01



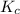 for this reaction is 0.088
for this reaction is 0.088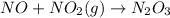
![K_c=\frac{[N_2O_3]}{[NO_2][NO]}](/tpl/images/0663/9574/d5725.png)