What would be the pH of an H2SO4 solution if the [H+] = 4.58 x 10-5 moles/liter?
9.66
4....

Chemistry, 22.05.2020 20:58 amelie1010
What would be the pH of an H2SO4 solution if the [H+] = 4.58 x 10-5 moles/liter?
9.66
4.34
5.67
NEXT QUESTION
3 ASK FOR HELP

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2
You know the right answer?
Questions


Physics, 20.05.2020 04:59


Mathematics, 20.05.2020 04:59

English, 20.05.2020 04:59

Physics, 20.05.2020 04:59






Mathematics, 20.05.2020 04:59


Biology, 20.05.2020 04:59

History, 20.05.2020 04:59

Social Studies, 20.05.2020 04:59

Mathematics, 20.05.2020 04:59

English, 20.05.2020 04:59

Mathematics, 20.05.2020 04:59

Advanced Placement (AP), 20.05.2020 04:59

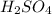 solution is 4.34
solution is 4.34![pH=-\log [H^+]](/tpl/images/0662/1654/37e81.png)
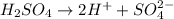
![pH=-\log[4.58\times 10^{-5}]](/tpl/images/0662/1654/0f1e2.png)
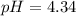
![[H^+]](/tpl/images/0662/1654/07acb.png) is
is ![4.58\times 10^{-5}]](/tpl/images/0662/1654/335c3.png) is 4.34
is 4.34

