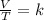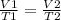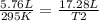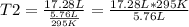Chemistry, 22.05.2020 05:05 jamaicalove2880
A 5.76 liter sample of a gas at 22.00C mL 748 torr pressure was heated to a final volume of 17.28 liters, with the pressure remaining constant. What was the final Celsius temperature ?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1
You know the right answer?
A 5.76 liter sample of a gas at 22.00C mL 748 torr pressure was heated to a final volume of 17.28 li...
Questions

Mathematics, 26.06.2019 16:00

Mathematics, 26.06.2019 16:00

English, 26.06.2019 16:00

Advanced Placement (AP), 26.06.2019 16:00


Chemistry, 26.06.2019 16:00

Computers and Technology, 26.06.2019 16:00

History, 26.06.2019 16:00

History, 26.06.2019 16:00


History, 26.06.2019 16:00


History, 26.06.2019 16:00

History, 26.06.2019 16:00

History, 26.06.2019 16:00



SAT, 26.06.2019 16:00