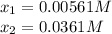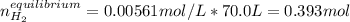Chemistry, 22.05.2020 02:10 Flameking1223
For the reaction
H2(g) + CO2(g) ⇆ H2O(g) + CO(g)
at 700°C, Kc = 0.534. Calculate the number of moles of H2 that are present at equilibrium if a mixture of 0.680 mole of CO and 0.680 mole of H2O is heated to 700°C in a 70.0−L container.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
You know the right answer?
For the reaction
H2(g) + CO2(g) ⇆ H2O(g) + CO(g)
at 700°C, Kc = 0.534. Calcu...
H2(g) + CO2(g) ⇆ H2O(g) + CO(g)
at 700°C, Kc = 0.534. Calcu...
Questions

Mathematics, 12.12.2020 17:00

Health, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

Advanced Placement (AP), 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


English, 12.12.2020 17:00

Chemistry, 12.12.2020 17:00

Health, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

English, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00




Health, 12.12.2020 17:00

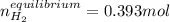
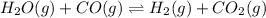
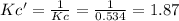
![Kc'=\frac{[H_2][CO_2]}{[H_2O][CO]}](/tpl/images/0660/7360/14ed3.png)
 due to the reaction extent:
due to the reaction extent:![Kc'=\frac{(x)(x)}{([H_2O]_0-x)([CO]_0-x)}=1.87](/tpl/images/0660/7360/2d81b.png)
![[H_2O]_0=[CO]_0=\frac{0.680mol}{70.0L}=0.0097M](/tpl/images/0660/7360/92f9f.png)