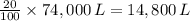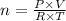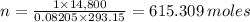Chemistry, 22.05.2020 01:06 micahsocool23
Assume the volume of the crew cabin is 74,000 L. The pressure is maintained at around 1.00 atm, ideally with an 80% nitrogen and 20% oxygen mix monitored by two gas systems. Assume that the temperature of the cabin is around 20ºC. How many moles of oxygen gas are present in the crew cabin at any given time? *

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

You know the right answer?
Assume the volume of the crew cabin is 74,000 L. The pressure is maintained at around 1.00 atm, idea...
Questions




Advanced Placement (AP), 18.08.2021 20:50





English, 18.08.2021 20:50



Chemistry, 18.08.2021 20:50




Social Studies, 18.08.2021 20:50

Mathematics, 18.08.2021 20:50