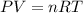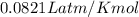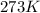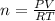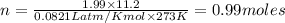Which sample would have the same number of molecules as 11.2L of He (g) at 273K and 202kPa?
1...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
Questions


Social Studies, 14.01.2021 17:00




Social Studies, 14.01.2021 17:00

Mathematics, 14.01.2021 17:00

History, 14.01.2021 17:00





Mathematics, 14.01.2021 17:00



Mathematics, 14.01.2021 17:00

History, 14.01.2021 17:00


History, 14.01.2021 17:00

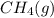 at 273K and 202kPa
at 273K and 202kPa