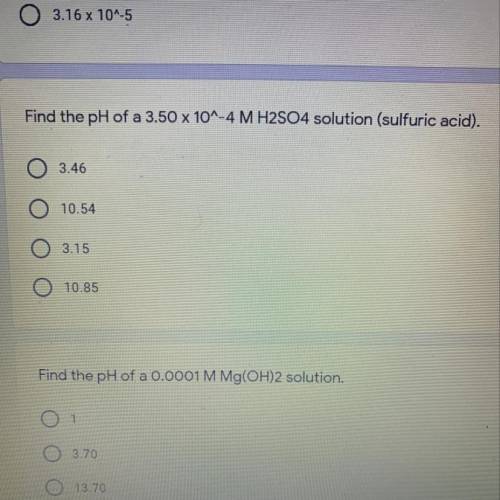
Find the pH of a 3.50 x 10^-4 M H2SO4 solution (sulfuric acid).
...

Chemistry, 20.05.2020 07:58 tzartiger12
Find the pH of a 3.50 x 10^-4 M H2SO4 solution (sulfuric acid).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Questions


Mathematics, 04.07.2019 01:00

Mathematics, 04.07.2019 01:00



History, 04.07.2019 01:00

Mathematics, 04.07.2019 01:00


Mathematics, 04.07.2019 01:00

Chemistry, 04.07.2019 01:00


Mathematics, 04.07.2019 01:00




Mathematics, 04.07.2019 01:00

History, 04.07.2019 01:00

History, 04.07.2019 01:00

Biology, 04.07.2019 01:00

English, 04.07.2019 01:00



