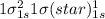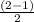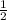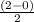Chemistry, 21.05.2020 05:10 korireidkdotdot82021
Use molecular orbital theory to determine whether He2 2+ or He2 + is more stable. Use molecular orbital theory to determine whether He2 2+ or He2 + is more stable. The He2 + ion is more stable since it has a higher bond order (bond order = 1) than the He2 2+ ion (bond order = 1/2). The He2 + ion is more stable since it has a higher bond order (bond order = 2) than the He2 2+ ion (bond order = 1). The He2 2+ ion is more stable since it has a lower bond order (bond order = 1/2) than the He2 + ion (bond order = 1). The He2 2+ a lower bond order (bond order = 3/2) than the He2 + ion (bond order = 2). The He2 2+ ion is more stable since it has a higher bond order (bond order = 1) than the He2 + ion (bond order = 1/2).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

You know the right answer?
Use molecular orbital theory to determine whether He2 2+ or He2 + is more stable. Use molecular orbi...
Questions


Mathematics, 18.11.2020 17:50


Chemistry, 18.11.2020 17:50


Mathematics, 18.11.2020 17:50


Mathematics, 18.11.2020 17:50

Computers and Technology, 18.11.2020 17:50

Physics, 18.11.2020 17:50

Arts, 18.11.2020 17:50

Mathematics, 18.11.2020 17:50

English, 18.11.2020 17:50


Chemistry, 18.11.2020 17:50