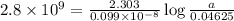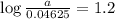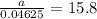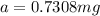Chemistry, 21.05.2020 01:08 genyjoannerubiera
If 0.04625 mg. of Uranium-235 remains after 2.8 x 10^9 years, what was
the original mass of the sample of Uranium-235? The half-life of Uranium-235 is 7.0 x 10^8 years.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
If 0.04625 mg. of Uranium-235 remains after 2.8 x 10^9 years, what was
the original mass of th...
the original mass of th...
Questions

Advanced Placement (AP), 29.10.2019 22:31



Mathematics, 29.10.2019 22:31

Mathematics, 29.10.2019 22:31

Mathematics, 29.10.2019 22:31


Computers and Technology, 29.10.2019 22:31


Mathematics, 29.10.2019 22:31


Mathematics, 29.10.2019 22:31


History, 29.10.2019 22:31


Mathematics, 29.10.2019 22:31



Mathematics, 29.10.2019 22:31

Advanced Placement (AP), 29.10.2019 22:31

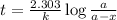
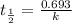
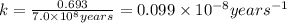
 years
years