
Chemistry, 21.05.2020 03:09 babygirl62716
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous methane and gaseous water in a 0.669 L flask at 1,020 K. At equilibrium, the flask contains 0.276 mol of CO gas, 0.207 mol of H2 gas, and 0.231 mol of methane. What is the water concentration at equilibrium (Kc = 0.30 for this process at 1,020 K)? Enter to 4 decimal places. HINT: Look at sample problem 17.7 in the 8th ed Silberberg book. Write a balanced chemical equation. Write the Kc expression. Calculate the equilibrium concentrations of all the species given (moles/liter). Put values into Kc expression, solve for the unknown.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous methane an...
Questions


Geography, 26.10.2019 15:43


Biology, 26.10.2019 15:43

Mathematics, 26.10.2019 15:43

History, 26.10.2019 15:43

Physics, 26.10.2019 15:43



English, 26.10.2019 15:43


English, 26.10.2019 15:43

English, 26.10.2019 15:43

Social Studies, 26.10.2019 15:43

Mathematics, 26.10.2019 15:43



Mathematics, 26.10.2019 15:43


Geography, 26.10.2019 15:43

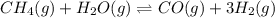
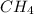 , [
, [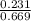 M = 0.345 M
M = 0.345 M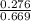 M = 0.413 M
M = 0.413 M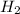 , [
, [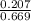 M = 0.309 M
M = 0.309 M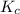 is expressed as:
is expressed as: ![K_{c}=\frac{[CO][H_{2}]^{3}}{[CH_{4}][H_{2}O]}](/tpl/images/0658/4057/27a18.png)
![\Rightarrow [H_{2}O]=\frac{[CO][H_{2}]^{3}}{[CH_{4}].K_{c}}=\frac{(0.413)\times (0.309)^{3}}{(0.345)\times (0.30)}= 0.1177](/tpl/images/0658/4057/4d76c.png)


