Chemistry, 21.05.2020 01:08 icantdomath6303
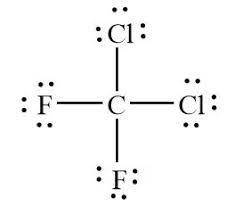
3. Most refrigerants utilize the energy involved in the liquid/gas phase change of a molecule, where an ideal refrigerant is noncorrosive, nonflammable, and has a boiling point around the desired temperature. Many fluorinated carbons meet all of these requirements, but the extremely high stability of some (such as CFCs) caused them to be phased out by the Montreal Protocol due to their ozone depleting potential. 3.a. R-12 (CCl2F2) was commonly used in household refrigerators and vehicle air conditioners prior to 1994. What is the correct Lewis structure for R-12

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2


Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
3. Most refrigerants utilize the energy involved in the liquid/gas phase change of a molecule, where...
Questions

Mathematics, 26.04.2021 20:50







Mathematics, 26.04.2021 20:50




Biology, 26.04.2021 20:50


Mathematics, 26.04.2021 20:50


Mathematics, 26.04.2021 20:50




Mathematics, 26.04.2021 20:50