
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a 500. mL flask with 3.7 atm of sulfur dioxide gas and 2.3 atm of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 2.2 atm. Calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions


Mathematics, 24.02.2020 23:24

Mathematics, 24.02.2020 23:24

Mathematics, 24.02.2020 23:24

Mathematics, 24.02.2020 23:24

English, 24.02.2020 23:24

Mathematics, 24.02.2020 23:24





Mathematics, 24.02.2020 23:24

Biology, 24.02.2020 23:24

Mathematics, 24.02.2020 23:24



Mathematics, 24.02.2020 23:24



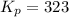
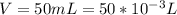
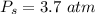
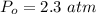
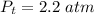
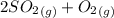 ⇄
⇄ 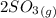
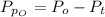
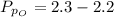
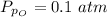
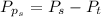
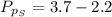
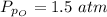
![K_p = \frac{[P_t]^2}{[P_p__{o}} ]^2 [P_p__{s}}]}](/tpl/images/0656/7414/8f8eb.png)
![K_p = \frac{[2.2]^2}{[ 0.1 ]^2 [{ 1.5}]}](/tpl/images/0656/7414/2f87d.png)



