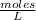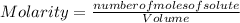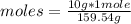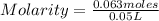Chemistry, 19.05.2020 03:20 smithsa10630
What is the molariity of a 50.0 mL aqueous solution containing 10.0 grams of copper (II) sulfate, CuSO4?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
What is the molariity of a 50.0 mL aqueous solution containing 10.0 grams of copper (II) sulfate, Cu...
Questions

Mathematics, 22.07.2020 04:01


Mathematics, 22.07.2020 04:01


Mathematics, 22.07.2020 04:01



Computers and Technology, 22.07.2020 04:01


Mathematics, 22.07.2020 04:01




History, 22.07.2020 04:01


Mathematics, 22.07.2020 04:01