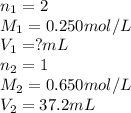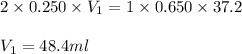Chemistry, 19.05.2020 03:15 evanwall91
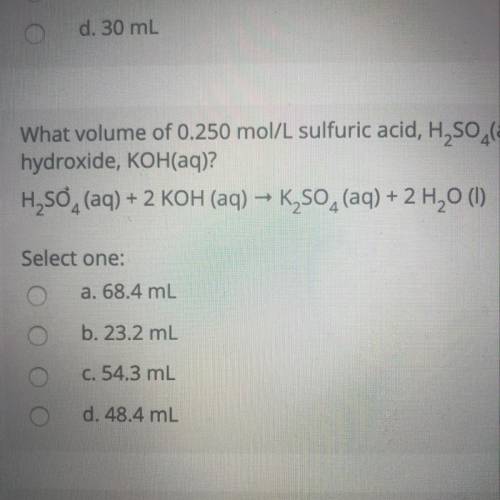
What volume of 0.250 mol/L sulfuric acid, H2SO4(aq) is needed to react completely with 37.2 mL of 0.650 mol/L potassium hydroxide, KOH(aq)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
What volume of 0.250 mol/L sulfuric acid, H2SO4(aq) is needed to react completely with 37.2 mL of 0....
Questions





Social Studies, 22.10.2019 21:20


History, 22.10.2019 21:20



Mathematics, 22.10.2019 21:20



Mathematics, 22.10.2019 21:20

Social Studies, 22.10.2019 21:20

Computers and Technology, 22.10.2019 21:20

English, 22.10.2019 21:20


History, 22.10.2019 21:20

Social Studies, 22.10.2019 21:20

Biology, 22.10.2019 21:20

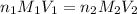
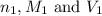 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 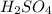
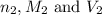 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.