
Chemistry, 19.05.2020 03:01 kayla32213
Methane, CH 4 , burns in oxygen to give carbon dioxide and water according to the following equation:
CH 4 + 2 O 2 > CO 2 + 2 H 2 O
In one experiment, a mixture of 0.250 mol of methane was burned in 1.25 mol of oxygen in a sealed steel
vessel. Find the limiting reactant and excess reactants.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
Methane, CH 4 , burns in oxygen to give carbon dioxide and water according to the following equation...
Questions


Business, 03.08.2019 13:30


Advanced Placement (AP), 03.08.2019 13:30



Advanced Placement (AP), 03.08.2019 13:30

English, 03.08.2019 13:30






Spanish, 03.08.2019 13:30


Mathematics, 03.08.2019 13:30

Mathematics, 03.08.2019 13:30



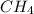 is the limiting reagent and
is the limiting reagent and 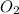 is the excess reagent.
is the excess reagent.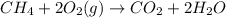
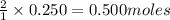 of
of 


