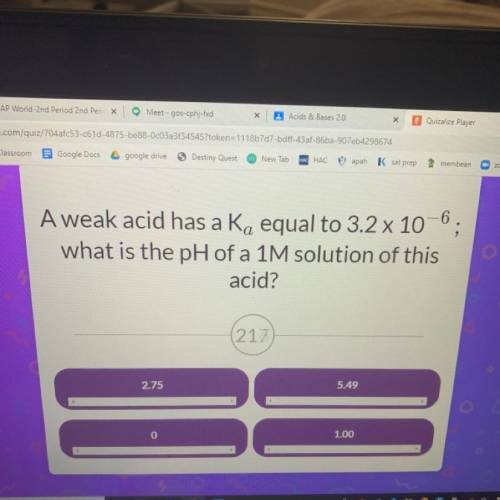
A weak acid has a Ka equal to 3.2 x 10^-6 what is the pH of a 1M solution of this acid?
...

Chemistry, 16.05.2020 11:57 khalidalrasheedi2025
A weak acid has a Ka equal to 3.2 x 10^-6 what is the pH of a 1M solution of this acid?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Questions


Mathematics, 22.08.2019 00:30

Social Studies, 22.08.2019 00:30

Mathematics, 22.08.2019 00:30


Geography, 22.08.2019 00:30

Business, 22.08.2019 00:30



English, 22.08.2019 00:30

Biology, 22.08.2019 00:30


History, 22.08.2019 00:30

Mathematics, 22.08.2019 00:30



Biology, 22.08.2019 00:30


English, 22.08.2019 00:30

Mathematics, 22.08.2019 00:30



