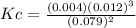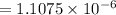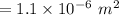Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2
You know the right answer?
FOR PRACTICE 15.6 The reaction
of CH4 in Example 15.6 is carried
out at a different temp...
of CH4 in Example 15.6 is carried
out at a different temp...
Questions

Biology, 23.11.2019 02:31

Biology, 23.11.2019 02:31

Biology, 23.11.2019 02:31

Physics, 23.11.2019 02:31


Biology, 23.11.2019 02:31


Social Studies, 23.11.2019 02:31


Social Studies, 23.11.2019 02:31


Health, 23.11.2019 02:31








![Kc(Equilibrium \ constant)=\frac{[C2H2][H2]^3}{[CH4]^2}](/tpl/images/0653/5442/54ef8.png)