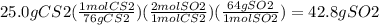4) When carbon disulfide burns in the presence of oxygen, sulfur dioxide and carbon
dioxide ar...

Chemistry, 09.05.2020 01:57 hannahbannana98
4) When carbon disulfide burns in the presence of oxygen, sulfur dioxide and carbon
dioxide are produced according to the following equation:
CS2 + 3 O2 → CO2 + 2 SO2
a) What is the percent yield of sulfur dioxide if the burning of 25.0 g of carbon
disulfide produces 40.5 g of sulfur dioxide?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
Questions




Mathematics, 25.06.2019 21:30

History, 25.06.2019 21:30


Mathematics, 25.06.2019 21:30


Mathematics, 25.06.2019 21:30

Computers and Technology, 25.06.2019 21:30




Mathematics, 25.06.2019 21:30

History, 25.06.2019 21:30


Mathematics, 25.06.2019 21:30