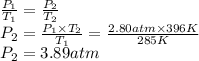Chemistry, 07.05.2020 11:01 BrandonBBB
What would the pressure be if 2.80atm of air is put into a 15.6L fixed volume cylinder and heated from 285K to 396K?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
What would the pressure be if 2.80atm of air is put into a 15.6L fixed volume cylinder and heated fr...
Questions


Mathematics, 01.03.2020 08:50




History, 01.03.2020 08:54




Mathematics, 01.03.2020 08:55

Mathematics, 01.03.2020 08:55




Arts, 01.03.2020 08:58

Physics, 01.03.2020 09:00

Chemistry, 01.03.2020 09:01