I NEED HELP NOW YOU CAN TAKE ALL MY POINTS JUST PLEASE ANSWER AND MAKE SURE ITS GOOD
Bel...

I NEED HELP NOW YOU CAN TAKE ALL MY POINTS JUST PLEASE ANSWER AND MAKE SURE ITS GOOD
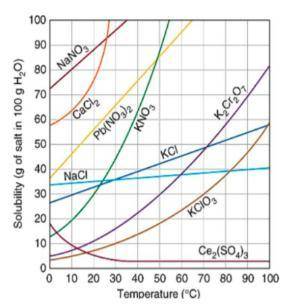
Below is a solubility curve to help you answer the following questions.
Another student is attempting to make a saturated solution of KCl. The student added 30 g of KCl to 100 g of water at 60℃. What more does the student need to do to create a saturated KCl solution?
Claim: What more does the student need to do to create a saturated KCl solution?
Evidence: Use data from the solubility curve to identify the number of grams of KCl needed at 60℃ to create a saturated solution.
Reasoning: Use your knowledge of the relationship between temperature and solubility to explain how you determined the saturation level of the solution at the given temperature. Be sure to define the terms you used.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
Questions


Health, 06.10.2019 04:00





Computers and Technology, 06.10.2019 04:00








Chemistry, 06.10.2019 04:01








