
The cell potential for the cell Zn(s) + 2H+(? M) LaTeX: \longrightarrow⟶ Zn2+(1.3 M) + H2(g) (8 atm) is observed to be 0.68 V. What is the pH in the H+/H2 half-cell? Reduction potential for H2(g)/H+(aq) is 0.00 V, for Zn(s)/Zn2+(aq) is -0.76 V. Enter number to 2 decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
The cell potential for the cell Zn(s) + 2H+(? M) LaTeX: \longrightarrow⟶ Zn2+(1.3 M) + H2(g) (8 atm)...
Questions

English, 28.01.2021 22:10

Health, 28.01.2021 22:10

Law, 28.01.2021 22:10

Mathematics, 28.01.2021 22:10



History, 28.01.2021 22:10



Mathematics, 28.01.2021 22:10

History, 28.01.2021 22:10


History, 28.01.2021 22:10

Mathematics, 28.01.2021 22:10

Mathematics, 28.01.2021 22:10

Mathematics, 28.01.2021 22:10

Mathematics, 28.01.2021 22:10



Social Studies, 28.01.2021 22:10

 half cell is 0.84.
half cell is 0.84.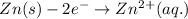
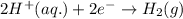
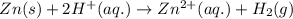
 = (0.00 V) + (0.76 V) = 0.76 V
= (0.00 V) + (0.76 V) = 0.76 V![E_{cell}=E_{cell}^{0}-\frac{0.0592}{n}log\frac{[Zn^{2+}].P_{H_{2}}}{[H^{+}]^{2}}](/tpl/images/0651/7884/0ba03.png)
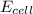 is cell potential, n is number of electron exchanged,
is cell potential, n is number of electron exchanged,  is pressure of
is pressure of  in atm and species under third bracket represent molarity of the respective species.
in atm and species under third bracket represent molarity of the respective species.![0.68V=0.76V-\frac{0.0592}{2}log\frac{(1.3M)\times (8atm)}{[H^{+}]^{2}}V](/tpl/images/0651/7884/ff1b4.png)

![[H^{+}]=0.1436M](/tpl/images/0651/7884/722f7.png)
![-log[H^{+}]](/tpl/images/0651/7884/1d5a1.png) = -log(0.1436) = 0.84
= -log(0.1436) = 0.84

