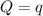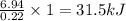Chemistry, 07.05.2020 05:16 rosepetals2938
The energy content of food is typically determined using a bomb calorimeter. Consider the combustion of a 0.22-g sample of butter in a bomb calorimeter having a heat capacity of 2.67 kJ/°C. If the temperature of the calorimeter increases from 23.5°C to 26.1°C, calculate the energy of combustion per gram of butter. Energy of combustion = kJ/g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
The energy content of food is typically determined using a bomb calorimeter. Consider the combustion...
Questions


Mathematics, 13.07.2019 02:30

Biology, 13.07.2019 02:30




Physics, 13.07.2019 02:30

History, 13.07.2019 02:30



English, 13.07.2019 02:30

Mathematics, 13.07.2019 02:30

Social Studies, 13.07.2019 02:30



Mathematics, 13.07.2019 02:30

Mathematics, 13.07.2019 02:30



History, 13.07.2019 02:30

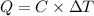

 =
= 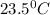
 =
=