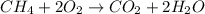Chemistry, 06.05.2020 23:07 Trucofer2106
Acetylene gas (C2H2) reacts with oxygen to produce carbon dioxide and water. When 30.0 g of acetylene is reacted with O2, 18.5 g of water is formed.
2 C2H2 (g) + 3 O2 (g) → 2 CO2 (g) + 2 H2O (l)
What type of reaction is this?
What is the percent yield of this reaction?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1


Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
Acetylene gas (C2H2) reacts with oxygen to produce carbon dioxide and water. When 30.0 g of acetylen...
Questions









Mathematics, 17.07.2019 20:10



History, 17.07.2019 20:10



Computers and Technology, 17.07.2019 20:10

Computers and Technology, 17.07.2019 20:10

Mathematics, 17.07.2019 20:10

Mathematics, 17.07.2019 20:10