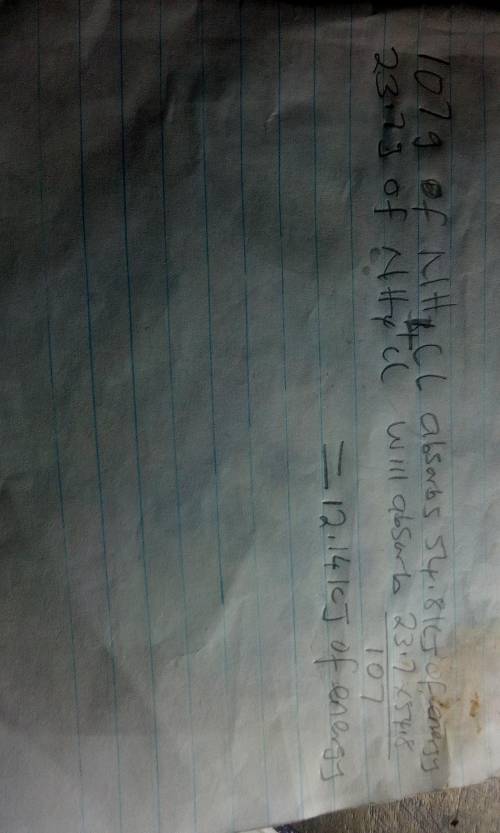Chemistry, 03.02.2020 20:45 PrincessIndia
The energy diagram shown represents the chemical reaction between solid ammonium chloride and solid barium hydroxide octahydrate:
2nh4cl(s) + ba(oh)2 x 8h2o(> 2nh3(aq)+bacl2(aq)+10h2o(1)
the (triangle then an h) for this reaction is 54.8 kj. how much energy would be absorbed if 23.7 g of nh4cl reacts?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2


Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
The energy diagram shown represents the chemical reaction between solid ammonium chloride and solid...
Questions






Mathematics, 28.02.2020 22:46








Health, 28.02.2020 22:46