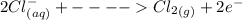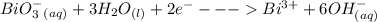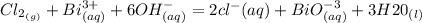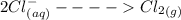Write balanced half-reactions for the following redox reactions
Cl2(g) + Bi3+ (aq) + 6OH-(aq)...

Chemistry, 06.05.2020 20:11 owlette2001
Write balanced half-reactions for the following redox reactions
Cl2(g) + Bi3+ (aq) + 6OH-(aq) = 2cl-(aq) + BiO-3 (aq) + 3H20 (l)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1
You know the right answer?
Questions


History, 26.07.2019 02:00






History, 26.07.2019 02:00

Health, 26.07.2019 02:00

Social Studies, 26.07.2019 02:00

Mathematics, 26.07.2019 02:00

Social Studies, 26.07.2019 02:00

Business, 26.07.2019 02:00



Mathematics, 26.07.2019 02:00




English, 26.07.2019 02:00