
Chemistry, 06.05.2020 03:32 RealSavage4Life
Consider these generic half-reactions. Half-reaction E° (V) X+(aq)+e−⟶X(s) 1.52 Y2+(aq)+2e−⟶Y(s) −1.17 Z3+(aq)+3e−⟶Z(s) 0.84 Identify the strongest oxidizing agent. X+ Y2+ X Z Y Z3+ Identify the weakest oxidizing agent. X Z3+ Y Y2+ Z X+ Identify the strongest reducing agent. Z3+ X+ Y2+ Z X Y Identify the weakest reducing agent. Y Z X+ X Y2+ Z3+ Which substances can oxidize Z ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2


Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
Consider these generic half-reactions. Half-reaction E° (V) X+(aq)+e−⟶X(s) 1.52 Y2+(aq)+2e−⟶Y(s) −1....
Questions

Chemistry, 16.07.2019 01:10

Chemistry, 16.07.2019 01:10









Mathematics, 16.07.2019 01:10


Mathematics, 16.07.2019 01:10



Mathematics, 16.07.2019 01:10



Chemistry, 16.07.2019 01:10

Mathematics, 16.07.2019 01:10

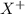
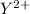
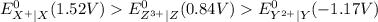
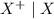 is higher than the reduction potential of the half cell
is higher than the reduction potential of the half cell 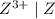 therefore
therefore 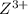 and itself gets converted into X.
and itself gets converted into X.

