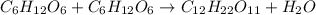Consider the following reaction.
c6h12o6+c6h12o6 -- c12h22o11
a. the equat...

Chemistry, 12.01.2020 01:31 mikayleighb2019
Consider the following reaction.
c6h12o6+c6h12o6 -- c12h22o11
a. the equation is not balanced; it is missing a molecule of water. write it in on the correct side of the equation.
b. what kind of reaction is this?
c. is c6h12o6 (glucose) a monomer, or a polymer?
d. to summarize, when two monomers are joined, a molecule of is always removed.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1

You know the right answer?
Questions

Mathematics, 22.07.2019 06:10



Physics, 22.07.2019 06:10

Biology, 22.07.2019 06:10

Physics, 22.07.2019 06:10


Mathematics, 22.07.2019 06:20





Health, 22.07.2019 06:20

Mathematics, 22.07.2019 06:20

Advanced Placement (AP), 22.07.2019 06:20

Mathematics, 22.07.2019 06:20


Mathematics, 22.07.2019 06:20

Biology, 22.07.2019 06:20