
Chemistry, 06.05.2020 01:35 cbelew0001ouje4i
Which of the following item(s) explain the differences between the Ka values. Choose one or more: A. The oxidation state for oxygen in trifluoroacetate is more negative than the oxidation state for oxygen in acetate. B. The trifluoroacetate molecule has more resonance structures than the acetate molecule. C. The electron-withdrawing fluorine atoms pull electron density from the oxygen in trifluoroacetate. The negative charge is more stabilized in trifluoroacetate by this effect. D. The negative charge is on the more electronegative fluorine atom in trifluoroacetate.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 23.06.2019 19:00
X-rays are used instead of visible light because x-rays have shorter than visible light, allowing them to produce images of much greater detail. a) energies b) frequencies c) speeds d) wavelengths
Answers: 3

Chemistry, 23.06.2019 23:00
Water molecules are polar with ends that exhibit partial positive and negative charges. such opposite charges make water molecules attract each other through which type of bonds?
Answers: 1
You know the right answer?
Which of the following item(s) explain the differences between the Ka values. Choose one or more: A....
Questions



Mathematics, 20.10.2019 07:30


Advanced Placement (AP), 20.10.2019 07:30

Biology, 20.10.2019 07:30

Mathematics, 20.10.2019 07:30


Computers and Technology, 20.10.2019 07:30



English, 20.10.2019 07:30

History, 20.10.2019 07:30


Social Studies, 20.10.2019 07:30

Biology, 20.10.2019 07:30




Social Studies, 20.10.2019 07:30

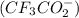 ,The acetate anion Two oxygen atoms.
,The acetate anion Two oxygen atoms.


