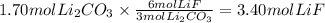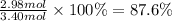The chemical reaction between lithium carbonate and aluminum trifluoride produces aluminum carbonate and lithium fluoride:
2AlF3 + 3Li2CO3 → Al2(CO3)3 + 6LiF.
You have an excess of aluminum trifluoride and 1.70 moles of lithium carbonate, which produces 2.98 moles of lithium fluoride. What is the percent yield of the reaction? Use the periodic table and this polyatomic ion resource.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 23.06.2019 10:30
If a computer chip switches off -on-off in 0.015 us, what is the switching time in nanoseconds?
Answers: 2

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2

Chemistry, 23.06.2019 11:40
Which of the following observations indicates that an atom has neutrons? some uncharged particles are scattered by a beryllium atom when it hits a gold foil. some uncharged particles bounce back from a gold foil when it is bombarded with alpha particles. a radiation consisting of uncharged particles is emitted when alpha particles strike beryllium atoms. a radiation which attracts electrons is produced when a beryllium atom is bombarded with alpha particles.
Answers: 2
You know the right answer?
The chemical reaction between lithium carbonate and aluminum trifluoride produces aluminum carbonate...
Questions

Mathematics, 19.08.2020 01:01

History, 19.08.2020 01:01

Mathematics, 19.08.2020 01:01



Mathematics, 19.08.2020 01:01

Chemistry, 19.08.2020 01:01

Mathematics, 19.08.2020 01:01





Biology, 19.08.2020 01:01

Mathematics, 19.08.2020 01:01