
Chemistry, 05.05.2020 19:42 punkinrichard1oxon2i
A student does a titration with 14.80 mL of 0.225M H2SO4 and 25.00 mL of unknown LiOH solution. What is the molarity of the base Pls hurry

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1


Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2
You know the right answer?
A student does a titration with 14.80 mL of 0.225M H2SO4 and 25.00 mL of unknown LiOH solution. What...
Questions




Mathematics, 20.09.2020 03:01





Mathematics, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01


Mathematics, 20.09.2020 03:01

Biology, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01


Mathematics, 20.09.2020 03:01



Mathematics, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01

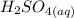 +
+ 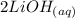 →
→ 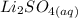 +
+ 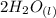
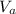 = 14.80 mL ,
= 14.80 mL , 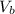 = 25.00 mL ,
= 25.00 mL , 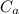 = 0.225M and
= 0.225M and 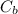 = ?
= ?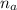 = 1 ,
= 1 , 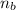 = 2
= 2

