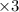Balance the redox reaction below using the half-reaction method. Sn2+(aq) + In(s)Sn(s) + In3+(aq) (a) To show your method, write the balanced half reactions below. Use the smallest integer coefficients possible and show electrons as e- . If a box is not needed, leave it blank. (Coefficients of 1 are not needed). Oxidation half-reaction: + + Reduction half-reaction: + + (b) To show your balanced equation, enter an integer in each of the boxes. If the integer is "1," do enter it even though you would normally not show that in the equation. Use the smallest integer coefficients possible. Sn2+(aq) + In(s) Sn(s) + In3+(aq)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
Balance the redox reaction below using the half-reaction method. Sn2+(aq) + In(s)Sn(s) + In3+(aq) (a...
Questions

English, 09.07.2019 19:30


Biology, 09.07.2019 19:30


Biology, 09.07.2019 19:30





English, 09.07.2019 19:30

Biology, 09.07.2019 19:30


Mathematics, 09.07.2019 19:30

Mathematics, 09.07.2019 19:30


English, 09.07.2019 19:30

English, 09.07.2019 19:30


Mathematics, 09.07.2019 19:30

Spanish, 09.07.2019 19:30

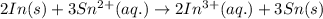
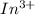 .
. 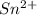 consume electrons and reduced to Sn.
consume electrons and reduced to Sn.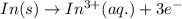 ]
]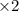
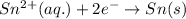 ]
]