
Chemistry, 05.05.2020 18:40 lillianneal
Substance ΔG°f(kJ/mol) M3O4(s) −8.80 M(s) 0 O2(g) 0 Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M3O4(s)↽−−⇀ 3M(s)+2O2(g) What is the standard change in Gibbs energy for the reaction, as written, in the forward direction? ΔG∘rxn= kJ/mol What is the equilibrium constant of this reaction, as written, in the forward direction at 298 K? K= What is the equilibrium pressure of O2(g) over M(s) at 298 K?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 13:10
When can a hypothesis be elevated to the status of a theory? a) when it is validated by an experiment b) when data gathered from an experiment precisely fits predictions c) when it can be proved to be true d) when it meets the test of repeated experimentation
Answers: 2

Chemistry, 23.06.2019 14:00
Ahas distinct properties and composition that never vary.
Answers: 1
You know the right answer?
Substance ΔG°f(kJ/mol) M3O4(s) −8.80 M(s) 0 O2(g) 0 Consider the decomposition of a metal oxide to i...
Questions


Social Studies, 17.10.2019 22:00



World Languages, 17.10.2019 22:00

English, 17.10.2019 22:00

Mathematics, 17.10.2019 22:00




Chemistry, 17.10.2019 22:00

History, 17.10.2019 22:00

History, 17.10.2019 22:00

Mathematics, 17.10.2019 22:00


Chemistry, 17.10.2019 22:00

Business, 17.10.2019 22:00



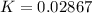
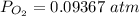
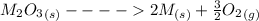
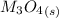 is
is 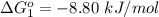
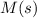 is
is 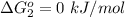
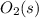 is
is 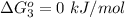
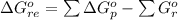
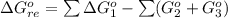
![\Delta G^o_{re} =[ (2 * 0) + (\frac{3}{2} * 0 )] - [1 * - 8.80]](/tpl/images/0641/3844/374c7.png)
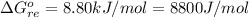

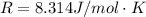
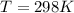
![K_p =[ P_{O_2}]^{\frac{3}{2} }](/tpl/images/0641/3844/66043.png)
![[ P_{O_2}]](/tpl/images/0641/3844/bc839.png) is the equilibrium pressure of oxygen
is the equilibrium pressure of oxygen 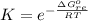
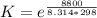
![0.02867 = [P_{O_2}]^{[\frac{3}{2} ]}](/tpl/images/0641/3844/d1a4b.png)
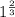
![P_{O_2} = [0.02867]^{\frac{2}{3} }](/tpl/images/0641/3844/dba69.png)


