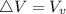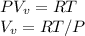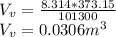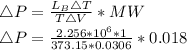Starting at atmospheric pressure, by how much must the pressure change in order to lower the boiling point of water by 1° C? You may assume that these changes are both small, so you only need to compute first derivatives. Remember that, under most conditions, the volume (per molecule) of liquid water is small compared to that of water vapor. Remember: Atmospheric pressure = 101300 Pa Boiling point at atmospheric pressure = 373.15 K Latent heat (LB) of boiling water = 2.256 × 106 J/kg Molecular weight (mw) of water = 0.018 kg/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 23.06.2019 14:30
An atom of element x has one more shell of electrons than an atom of beryllium, but it has one less valance electron than beryllium. which element is x
Answers: 1
You know the right answer?
Starting at atmospheric pressure, by how much must the pressure change in order to lower the boiling...
Questions


Biology, 12.04.2020 19:47


Chemistry, 12.04.2020 19:47



Mathematics, 12.04.2020 19:47


English, 12.04.2020 19:47


Biology, 12.04.2020 19:47

Social Studies, 12.04.2020 19:47

English, 12.04.2020 19:47

Mathematics, 12.04.2020 19:47

Mathematics, 12.04.2020 19:47


Mathematics, 12.04.2020 19:48



Mathematics, 12.04.2020 19:48

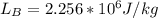
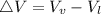
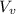 ) is far greater than the volume of liquid (
) is far greater than the volume of liquid (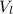 )
)