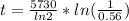Chemistry, 05.05.2020 17:41 tyresharichardson29
The ratio of carbon-14 to carbon-12 in the shaft of a wooden arrow, unearthed when a foundation was being dug for a new house, is 56.0% of the same ratio in a growing tree today. Assuming the ratio of carbon-14 to carbon-12 in the atmosphere has been constant, calculate the age of the arrow. The half-life of carbon-14 is 5730 years. The arrow is years old Numeric

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
The ratio of carbon-14 to carbon-12 in the shaft of a wooden arrow, unearthed when a foundation was...
Questions


Mathematics, 04.02.2020 01:46


Mathematics, 04.02.2020 01:46





Mathematics, 04.02.2020 01:46

Mathematics, 04.02.2020 01:46



Mathematics, 04.02.2020 01:46

Mathematics, 04.02.2020 01:46

Mathematics, 04.02.2020 01:46





Biology, 04.02.2020 01:46

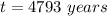 old
old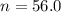 %
%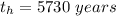
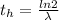
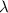 is the decay constant
is the decay constant 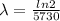
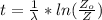
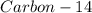 is
is 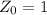
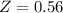 %
%